Analysis: The Draft NICE Guidelines
The National Institute for Health and Care Excellence (NICE) recently released its draft guidelines for prescribing cannabis-based medical products (CBMPs) in the UK. The release is significant because it is these guidelines - rather than politically-imposed regulations - that will effectively determine the extent of medical cannabis access on the NHS.
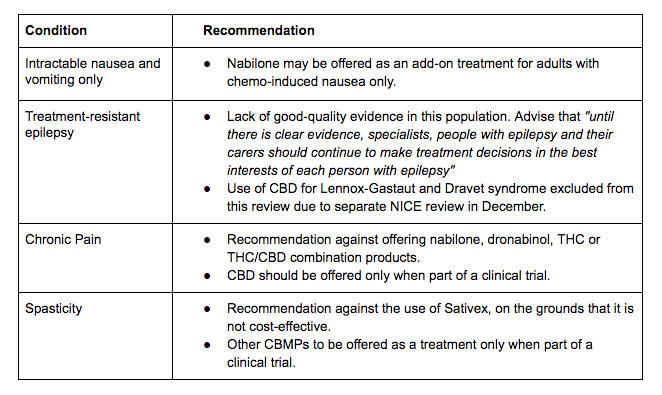
A summary of the proposed guidelines is given below. The final version of the document is due to be published by November following stakeholder feedback.
Prescribing recommendations:
The recommendations have disappointed those who hoped that the NICE guidelines would increase access to CBMPs - instead, the report largely echoes the limited recommendations in place today. In particular, the recommendation against CBMPs for the treatment of chronic pain is a bitter blow for the 13 percent of the UK adult population with the condition, and who may benefit from medical cannabis in some form. And while it is positive that the proposed guidelines allow epilepsy specialists to make CBMP prescribing decisions on a case-by-case basis, rather than recommending outright against their use, it is disappointing that such flexibility is not extended to additional conditions.
In total, the prescribing recommendations confirm a frustrating reality - that until either sufficient clinical data on cannabis’ efficacy is produced or the UK adopts an alternative framework for CBMP access, medical cannabis is likely to remain an option only for those who can afford up to £800 a month for a private prescription.
Shared-care arrangements
NICE acknowledges that the current requirement to see a specialist doctor for each CBMP prescription places an unfair burden on patients and carers. In response, they propose that repeat prescriptions could be issued by a non-specialist prescriber (such as a GP) as part of a properly-documented shared care agreement.
That NICE recognises the ‘clear need’ to streamline CBMP access is a good step in the right direction. Allowing greater involvement by non-specialist healthcare providers should reduce tertiary healthcare costs, increase exposure and comfort with medical cannabis throughout the healthcare profession, and hopefully pave the way towards greater autonomy for non-specialist healthcare providers - such as dose adjustment - as the scheme develops.
Research recommendations
Throughout the proposed guidelines, NICE is clear when a lack of good evidence limits the strength of their recommendations or prevents them from making one at all. NICE therefore proposes key research recommendations into five areas, including CBD as a treatment for persistent neuropathic pain, CBMPs for people with spasticity, and the effect of THC/CBD combination treatment on seizures. Research recommendations regarding intractable nausea and vomiting are also given, with research sought into both the cost-effectiveness of CBMPs as well as their clinical efficacy.
These recommendations are welcome and will help direct UK clinical trials and research where (in terms of expanding UK CBMP access) it is needed the most. By issuing several ‘priority’ recommendations NICE also signals a commitment to the rapid progression of such research, and facilitates the fast-tracking of project funding by the National Institute for Health Research (NIHR), the UK’s largest supporter of healthcare research.
Hanway’s analysis
Although disappointing, NICE’s limited recommendations are not unexpected and show that the major institutional hurdles to medical cannabis prescription are twofold. The first problem is a lack of clinical evidence, thanks to the decades-long restrictions on medical cannabis research. NICE’s research recommendations are a positive move, but given the high costs and long lead time for the RCTs typically required by NICE, as well as the potential for patients to source cannabis illegally when not faced with an affordable legal alternative, there are strong arguments for accepting an alternative burden of proof or prescription framework for CBMPs. Such avenues have been proposed by groups such as Transform and the Centre for Medical Cannabis while alternative study designs are even recommended by NHS England in the case of paediatric epilepsy. However, NICE cannot unilaterally lower the standard of evidence required for CBMPs or adopt a revised prescriptions framework: such direction must ultimately come from government.
The second key takeaway is that the high cost of medical cannabis reduces the likelihood of NHS access. NICE’s recommendations are driven by the cost-effectiveness of treatment rather than efficacy alone, and CBMPs fail to meet NICE’s affordability threshold at their current prices. For example, CBMPs would need to become either ten times cheaper or ten times more effective (or some combination of both) in order to be considered a cost-effective treatment for chronic pain. Similarly, NICE will continue to recommend against Sativex as a treatment for spasticity unless the purchase price falls from £375 to £188 per pack.
Affordability is an issue that industry should be actively working to address. Participants should set out how they intend to lower their costs, and explain any legal or regulatory barriers that prevent them from doing this effectively. Pricing for CBMPs excluded in NICE’s economic modelling (such as Bedrocan products and pure CBD oil) should also be shared to offer a more accurate view of the UK landscape.
Where cost is a problem, bulk supply and economies of scale can form part of the solution. For example, the availability of low-cost alternatives such as scaled production of cannabinoid APIs could radically shift affordability calculations, as could an increase in domestically-sourced products that avoid expensive import costs. And on the margin, working to lower CBMPs’ costs now will make them more affordable for patients who have few options between a private prescription or black-market cannabis.
Many in the industry have expressed disappointment with the NICE’s conservative view of the efficacy of medical cannabis and the limited CBMP access it recommends. But regardless of content, the guidelines are valuable for the clarity which they provide to doctors, prospective patients and the wider industry. Hanway Associates are submitting stakeholder feedback on the proposed guidelines, and are committed to working with regulators and politicians as well as industry to help build a sustainable and equitable medical cannabis framework for the UK.
For more insights and advice on cannabis policy developments in the UK, please get in touch at info@hanwayassociates.com.